SYSTEMATIC APPROACH TO THE EVALUATION OF ANEMIA
Figure 29-4 Approach to the differential diagnoses of anemia in the adult and child. G6PD, glucose-6-phosphate dehydrogenase; MCV, mean corpuscular volume; PNH, paroxysmal nocturnal hemoglobinuria; RDW, red blood cell distribution width.
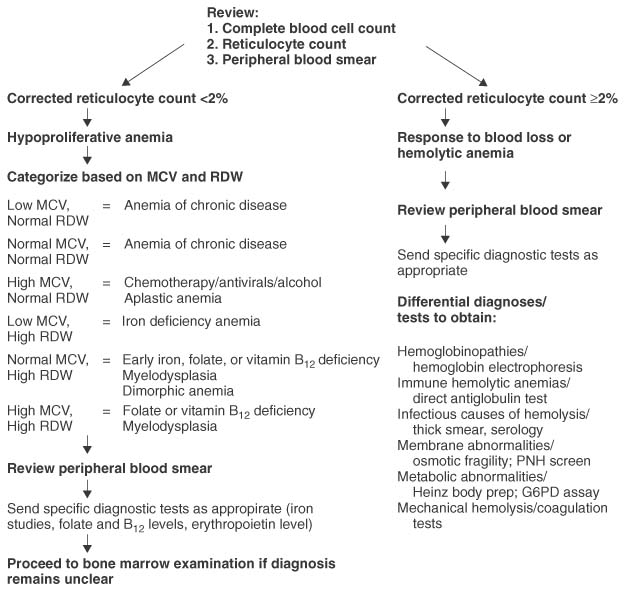
The correct diagnosis of anemia can often be determined by combining a thorough history and physical examination with thoughtful reviews of the complete blood cell count, reticulocyte count, and peripheral blood smear. Such an approach minimizes cost and the time to accurate diagnosis.
 Figure 29-2 Regulation of erythropoiesis. Figure 29-2 Regulation of erythropoiesis.
HISTORY AND PHYSICAL EXAMINATION
Because anemia can be a primary disorder or secondary to other systemic processes, a careful history and physical examination will provide valuable insight into the potential cause. Fatigue often accompanies anemia, but it is very nonspecific and may be related to systemic illness. Nonetheless, determining the concomitant presence of a systemic inflammatory disorder, infection, or malignancy associated with fatigue may be critical in determining the underlying causes of anemia in adults and children.[25] Past medical history may also be quite informative. For example, a history of diabetes mellitus can be associated with significantly impaired renal production of erythropoietin even in the setting of only a mildly elevated creatinine level.[26] Because certain medications may be associated with bone marrow depression or, alternatively, the development of autoimmune hemolytic anemia, all pharmacologic agents, prescribed and over-the-counter, including alternative medicines, should be reviewed.[27] Occupational history is occasionally relevant, as in the case of individuals, such as welders, who might have been exposed to lead or other potentially marrow toxic agents.[28] [29] Social history can be important, as a history of intravenous drug use might suggest the possibility of virally transmitted diseases, such as human immunodeficiency virus infection, that may be associated with anemia.[30] Dietary history is very important, particularly in the young and the elderly with anemia. The finding of pica in adults (most commonly ice chips or cornstarch) is well known to be associated with iron deficiency anemia.[31] Ingestion of paint chips may suggest the need to investigate the possibility of toxic lead ingestion. Family history of anemia is particularly important in the evaluation of children with anemia. However, it is also relevant in adults, because
TABLE 29-2 -- Normal Red Blood Cell Values
|
Hemoglobin g/dL |
Hematocrit (%) |
Red Cell Count (1012 /L) |
MCV (fL) |
MCH (pg) |
MCHC (g/dL) |
| Age |
Mean |
-2SD |
Mean |
-2SD |
Mean |
-2SD |
Mean |
-2SD |
Mean |
-2SD |
Mean |
-2SD |
| Birth (cord blood) |
16.5 |
13.5 |
51 |
42 |
4.7 |
3.9 |
108 |
98 |
34 |
31 |
33 |
30 |
| 1–3 days (capillary) |
18.5 |
14.5 |
56 |
45 |
5.2 |
4.0 |
108 |
95 |
34 |
31 |
33 |
29 |
| 1 week |
17.5 |
13.5 |
54 |
42 |
3.1 |
3.9 |
107 |
88 |
34 |
28 |
33 |
28 |
| 2 weeks |
16.5 |
12.5 |
51 |
39 |
4.9 |
3.6 |
105 |
86 |
34 |
28 |
33 |
28 |
| 1 month |
14.0 |
10.0 |
43 |
31 |
4.2 |
3.0 |
104 |
85 |
34 |
28 |
33 |
29 |
| 2 months |
11.5 |
9.0 |
35 |
28 |
3.8 |
2.7 |
96 |
77 |
30 |
26 |
33 |
29 |
| 3–6 months |
11.5 |
9.5 |
35 |
29 |
3.8 |
3.1 |
91 |
74 |
30 |
25 |
33 |
30 |
| 0.5–2 years |
12.0 |
11.0 |
36 |
33 |
4.5 |
3.7 |
78 |
70 |
27 |
23 |
33 |
30 |
| 2–6 years |
12.5 |
11.5 |
37 |
34 |
4.6 |
3.9 |
81 |
75 |
27 |
24 |
34 |
31 |
| 6–12 years |
13.5 |
11.5 |
40 |
35 |
4.6 |
4.0 |
86 |
77 |
29 |
25 |
34 |
31 |
| 12–18 years |
|
|
|
|
|
|
|
|
|
|
|
|
| Female |
14.0 |
12.0 |
41 |
36 |
4.6 |
4.1 |
90 |
78 |
30 |
25 |
34 |
31 |
| Male |
14.5 |
13.0 |
43 |
37 |
4.9 |
4.5 |
88 |
78 |
30 |
25 |
34 |
31 |
| 18–49 years |
|
|
|
|
|
|
|
|
|
|
|
|
| Female |
14.0 |
12.0 |
41 |
36 |
4.6 |
4.0 |
90 |
80 |
30 |
26 |
34 |
31 |
| Male |
15.5 |
13.5 |
47 |
41 |
5.2 |
4.5 |
90 |
80 |
30 |
26 |
34 |
31 |
| From Oski FA: Pallor. In Kaye R, Oski FA, Barness LA (eds): Core Textbook of Pediatrics, 3rd ed. Philadelphia, Lippincott, 1989, p 62. |
certain congenital anemias, such as hereditary spherocytosis, occasionally first manifest later in life.[32]
The significance of pallor on physical examination is in many ways similar to the historic feature of fatigue: it is a common yet non-specific finding. More specific findings may be found in certain types of anemias. For example, angular cheilitis (cracking at the edges of the lips) and koilonychia (spooning of the nails) may accompany iron deficiency anemia.[33] Splenomegaly may be present in patients with anemia arising from a wide variety of different causes.[34] When present early in life, it is suggestive of a congenital hemolytic anemia, such as thalassemia, sickle cell disease, or hereditary spherocytosis. When found for the first time later in life, splenomegaly may indicate an acquired disorder, such as autoimmune hemolytic anemia, lymphoproliferative disease, or agnogenic myeloid metaplasia. Other physical findings can also sometimes provide insight into the investigation of anemia when combined with historic features and laboratory data. Although anemia itself may lead to the presence of systolic cardiac murmurs, the finding of an increased cardiac murmur in an anemic patient with a prosthetic aortic valve and new microangiopathic change on peripheral smear may indicate that investigation into the possibility of perivalvular leak or prosthetic dysfunction is in order.[35] Finally, because neurologic manifestations can accompany or even predate the anemia associated with vitamin B12 deficiency, findings such as loss of vibration of position sense in the extremities may be relevant.[36]
RETICULOCYTE COUNT
As a marker of red blood cell production, the reticulocyte count provides important information in directing the initial investigation of anemia. Modern flow cytometers accurately determine the reticulocyte count using fluorescent probes that bind to the residual ribonucleic acid present in newly released red blood cells.[37] These measurements generally are useful, are accurate, and reflect the state of erythropoiesis. However, when significant numbers of nucleated red blood cells or nuclear debris are present in the peripheral blood, this diagnostic accuracy declines, and manual counting methods are generally preferable.
Because the reticulocyte count is often reported as a percentage, it needs to be adjusted for the total number of red blood cells present. This correction can be achieved by multiplying the reticulocyte count by the patient's hematocrit divided by an age- and gender-appropriate normal hematocrit. No such correction is necessary when the reticulocyte number is reported as an absolute number. Although additional correction factors can be used to correct for the presence of early reticulocytes (such as halving the reticulocyte count in the presence of polychromatophasia on peripheral smear), these are not routinely necessary. For practical purposes, corrected reticulocyte counts of less than 2% or absolute reticulocyte counts of less than 100,000/µL are associated with hypoproliferative anemias, whereas values above these are associated with either an appropriate response to blood loss or with hemolytic (hyperproliferative) anemias ( Table 29-3 ).
MEAN CORPUSCULAR VOLUME AND RED BLOOD CELL DISTRIBUTION WIDTH
Automated cell counters provide a wealth of information regarding the size, shape, and hemoglobin content of the red blood cell. The two parameters most useful in classifying anemia are the mean corpuscular volume (MCV) and the red blood cell distribution width (RDW). MCV is reported in femtoliters (fL) and reflects average cell size. RDW is a dimensionless quantity (actually the standard deviation of red blood cell volume divided by the mean volume) that reflects the variation in cell size in the population of red blood cells.[38] The usefulness of these parameters lies in the fact that relatively reproducible changes in them are observed in certain types of anemias ( Table 29-4 ). When combined with the reticulocyte count, the MCV and RDW can significantly narrow the differential diagnosis ( Table 29-5 ), thus facilitating thoughtful review of the peripheral blood smear.
EXAMINATION OF THE PERIPHERAL BLOOD SMEAR
Despite the availability of automated cell counters and sophisticated diagnostic testing, review of a well-made peripheral blood smear
TABLE 29-3 -- Usefulness of the Reticulocyte Count in the Diagnosis of Anemia
| Diagnosis |
Value |
| Hypoproliferative anemias |
Corrected reticulocyte count <2% or absolute reticulocyte count <100,000/µl |
| Anemia of chronic disease |
|
| Anemia of renal disease |
|
| Congenital dyserythropoietic anemias |
|
| Effects of drugs or toxins |
|
| Endocrine anemias |
|
| Iron deficiency |
|
| Marrow replacement |
|
| Maturation abnormalities |
|
| Vitamin B12 deficiency |
|
| Folate deficiency |
|
| Sideroblastic anemia |
|
| Appropriate response to blood loss or nutritional supplementation |
Corrected reticulocyte count ≥2% or absolute reticulocyte count >100,000/µl |
| Hemolytic anemias |
|
| Hemoglobinopathies |
|
| Immune hemolytic anemias |
|
| Infectious causes of hemolysis |
|
| Membrane abnormalities |
|
| Metabolic abnormalities |
|
| Mechanical hemolysis |
|
TABLE 29-4 -- Usefulness of the Mean Corpuscular Value (MCV) and Red Blood Cell Distribution Width (RDW) in the Diagnosis of Anemia
|
Low MCV (<80 fL) |
Normal MCV (80–99 fL) |
High MCV (>100 fL) |
| Normal RDW |
Anemia of chronic disease |
Acute blood loss |
Aplastic anemia |
|
α- or β-Thalassemia trait |
Anemia of chronic disease |
Chronic liver disease |
|
Hemoglobin E trait |
Anemia of renal disease |
Chemotherapy/antivirals/alcohol |
| Elevated RDW |
Iron deficiency |
Early iron, folate, or vitamin B12 deficiency |
Folate or vitamin B12 deficiency |
|
Sickle cell-β-thalassemia |
Dimorphic anemia (for example, iron + folate deficiency) |
Immune hemolytic anemia |
|
|
Sickle cell anemia |
Cytotoxic chemotherapy |
|
|
Sickle cell disease |
Chronic liver disease |
|
|
Chronic liver disease |
Myelodysplasia |
|
|
Myelodysplasia |
|
TABLE 29-5 -- Combining the Reticulocyte Count and Red Blood Cell Parameters for Diagnosis
|
Corrected Reticulocyte Count <2% |
Corrected Reticulocyte Count ≥2% |
| Low MCV, Normal RDW |
Anemia of chronic disease |
|
| Normal MCV, Normal RDW |
Anemia of chronic disease |
|
| High MCV, Normal RDW |
Chemotherapy/antivirals/alcohol |
Chronic liver disease |
|
Aplastic anemia |
|
| Low MCV, High RDW |
Iron deficiency anemia |
Sickle cell-β-thalassemia |
| Normal MCV, High RDW |
Early iron, folate, vitamin B12 deficiency |
Sickle cell anemia, sickle cell disease |
|
Myelodysplasia |
|
| High MCV, High RDW |
Folate or vitamin B12 deficiency |
Immune hemolytic anemia |
|
Myelodysplasia |
Chronic liver disease |
| MCV, mean corpuscular volume; RDW, red blood cell distribution width |
remains one of the most informative and rewarding diagnostic procedures. It offers the chance to confirm the findings of the automated complete blood cell count, which can be inaccurate in the presence of nucleated red blood cells or rouleaux formation. Review of the blood smear also allows for evaluation of other cell lineages, which might suggest a primary marrow or infiltrative disease. The finding of hypersegmented neutrophils suggests a megaloblastic process, and this morphologic abnormality can be seen in the blood smear before there are significant changes in the hemoglobin or MCV (see the box on the Systematic Approach to the Diagnosis of Anemia ). Also, only the blood smear reveals the unique morphologic changes occurring with various hemolytic disorders.
There are several commonly encountered findings that can be seen in red blood cells on the peripheral blood smear ( Table 29-6 ). Microcytic, hypochromic red blood cells are suggestive of iron deficiency anemia or thalassemia ( Fig. 29-3A ); whereas macrocytic red blood cells with ovalocytes (oval red blood cells) are suggestive of megaloblastic anemias (see Fig. 29-3B ). Some findings reflect organ dysfunction, such as echinocytes (burr cells) in uremia (see Fig. 29-3C ), or acanthocytes (spur cells) in severe liver disease (see Fig. 29-3D ), although acanthocytes may also be seen in rare conditions such as abetalipoproteinemia. Target cells may be seen in cases of liver disease but may also be present in hemoglobinopathies, including sickle cell disease or thalassemia (see Fig. 29-3E ). The presence of
schistocytes or red blood cell fragmentation usually reflects systemic disease, such as disseminated intravascular coagulation, thrombotic thrombocytopenic purpura, or the hemolytic-uremic syndrome (see Fig. 29-3F ). Finding spherocytes on a smear is suggestive of autoimmune hemolytic anemia or hereditary spherocytosis (see Fig. 29-3G ). Occasionally, the clue to the correct diagnosis of a systemic illness comes in the form of the observation of intraerythrocytic inclusions, such as malarial or babesial forms, and examination of a thick blood smear may be useful for the diagnosis of these disorders when a low parasite burden is suspected.
|
Systematic Approach to the Diagnosis of Anemia
Whether in the adult or the child, a systematic approach to the evaluation of anemia is most productive and can often avoid the need for expensive or invasive diagnostic tests. Integration of historic features and physical findings with thoughtful review of the results of the automated complete blood cell count and of the peripheral smear often serves to narrow down the differential diagnosis significantly. For example, a patient status post-gastric bypass eating a normal diet who presents with gradual onset of fatigue accompanied by the more recent onset of distal paresthesias and a finding of decreased vibration sense, in the setting of significantly elevated MCV and RDW values and numerous six-lobed polymorphonuclear leukocytes on peripheral blood smear, almost certainly has a vitamin B12 deficiency. This is suggested even before the return of specific laboratory testing because of the differential diagnosis list for megaloblastic anemia, which is further narrowed because neurologic abnormalities are not associated with folate deficiency. For diagnostic efficiency, the rewards of correlation of historic features and physical findings with a careful review of the peripheral blood smear cannot be overstated. |
Special stains of the peripheral blood smear can be helpful in elucidating the cause of anemia in certain cases. If there is significant nuclear debris present, the reticulocyte count obtained by automated methods can be inaccurate. In such cases, manual counting after staining with new methylene blue, which stains residual RNA in reticulocytes, permits accurate enumeration. If bites cells are detected on peripheral smear, supravital staining with methyl crystal violet can reveal Heinz bodies, aggregates of denatured hemoglobin reflecting an oxidative insult, due most commonly to glucose-6-phosphate dehydrogenase deficiency or, to a lesser extent, the presence of an unstable hemoglobin (see Fig. 29-3H ).
BONE MARROW EXAMINATION
Bone marrow aspiration and biopsy permit evaluation of cellular morphology and marrow architecture, respectively. Special stains, flow cytometry, and cytogenetics that can be performed on the marrow also can provide a wealth of diagnostic information.[39] Because of the discomfort involved in the procedure, however, careful consideration should be given to determining the array of tests required, so that repeated marrow aspirates or biopsies need not be performed. If there is any consideration of the possibility of myelodysplasia, leukemia, or lymphoma, an aliquot of anticoagulated aspirate should be set aside at the time of the initial procedure that can be sent, if necessary, for flow cytometry or cytogenetics after review of the aspirate smear.
Diagnostic uncertainty in the setting of hypoproliferative anemia is an indication for bone marrow biopsy. Hematologic disorders such as myelodysplasia, leukemia, lymphoma, or myeloma may be identified. Myelodysplasia in the marrow classically includes megaloblastic change and nuclear budding in maturing erythroblasts, as well as morphologic abnormalities in other lineages, such as hypolobated megakaryocytes and hypogranulation of the myeloid lineage.[40] A variety of infiltrative (myelophthisic) processes may be observed. These include malignancies such as small cell lung, breast, and prostate cancers, which not infrequently can appear in advanced stages with marrow involvement. Alternatively, granulomas may be present, suggesting the possible presence of mycobacterial disease. In children, disseminated neuroblastoma and rhabdomyosarcoma occasionally can appear as a myelophthisic anemia.
Even when properly performed, difficulty obtaining a bone marrow aspirate is commonly observed in certain situations, such as myelofibrosis, erythroblastic leukemia (M6), and hairy cell leukemia.[41] In these cases, touch preps of the bone marrow biopsy may help expedite diagnosis.
APPROACH TO HYPOPROLIFERATIVE ANEMIA IN THE ADULT
Hypoproliferative anemia is reasonably common in adults. Except in the setting of acute blood loss, the new onset of anemia in an adult is more commonly associated with a hypoproliferative process rather than a hemolytic one. A systematic approach integrating historic features with laboratory findings can often lead to a short differential diagnosis list ( Fig. 29-4 ).
Microcytic anemia with an MCV of less than 70fL is most commonly associated with iron deficiency anemia or thalassemia. Values between about 70fL and the lower limit of the normal range may be associated with the anemia of chronic disease, endocrine causes such as hyperthroidism, or other causes. Macrocytic anemias with an MCV of greater than 120fL are not infrequently associated with folate or vitamin B12 deficiency. Otherwise, MCV values above the upper limit of normal suggest the possibility of increased ethanol intake, liver disease, or bone marrow failure states such as aplastic anemia or myelodysplastic syndromes.
The most commonly encountered normocytic hypoproliferative anemia in adults is that of chronic disease, which, when broadly defined, includes anemias due to renal disease and inflammatory causes. In such cases, erythropoietin levels may be of value in the diagnostic evaluation, before proceeding to bone marrow examination. [42] Occasionally, a normocytic anemia represents the result of averaging two populations of red blood cells: one microcytic and one macrocytic. Such cases of dimorphic anemia are encountered in combined nutritional deficiencies, such as when iron and folate deficiencies are present. The RDW is usually markedly elevated in such cases, and review of the peripheral blood smear confirms the diversity in red blood cell size.
 Figure 29-3 A, Hypochromic microcytic anemia. B, Megaloblastic anemia. C, Burr cells. D, Spur cells. E, Target cells. F, Microangiopathic hemolytic anemia. G, Spherocytes. H, Heinz body hemolytic anemia. Figure 29-3 A, Hypochromic microcytic anemia. B, Megaloblastic anemia. C, Burr cells. D, Spur cells. E, Target cells. F, Microangiopathic hemolytic anemia. G, Spherocytes. H, Heinz body hemolytic anemia.
APPROACH TO HEMOLYTIC ANEMIA IN THE ADULT
Newly diagnosed hemolytic anemia is less common in adults than hypoproliferative anemia. Because the differential diagnosis is relatively broad, historical factors, such as information on the temporal nature of its development, and physical findings, such as splenomegaly, may help to narrow the diagnostic entities under consideration (see Fig. 29-4 ). Review of the peripheral blood smear is essential and potentially more useful than bone marrow examination in providing valuable diagnostic information.
Chronic hemolytic anemia in the adult may represent an inherited condition that has gone undiagnosed previously or may represent an acquired disorder. For example, mild to moderate hereditary spherocytosis or sickle/β+ -thalassemia intermedia may not manifest until adulthood. Autoimmune hemolytic anemia is not infrequently
TABLE 29-6 -- Features of the Peripheral Blood Smear
| Red Blood Cell Morphology |
Definition |
Interpretation |
| Polychromasia |
Large, bluish red blood cells lacking normal central pallor on peripheral blood smear; bluish stain is the result of residual ribonucleic acid. |
Rapid production and release of red blood cells from marrow; elevated reticulocyte count; most commonly seen in hemolytic anemia. |
| Basophilic stippling |
Many small bluish dots in portion of erythrocytes; comes from staining of clustered polyribosomes in young circulating red blood cells. |
Seen in a variety of erythropoietic disorders, including acquired and congenital hemolytic anemias and occasionally in lead poisoning (lead inhibits pyrimidine 5'-nucleotidase, which normally digests the residual RNA). |
| Pappenheimer bodies |
Several grayish, irregularly shaped inclusions in a portion of erythrocytes visible on peripheral smear; composed of aggregates of ribosomes, ferritin, and mitochondria. |
Erythropoietic malfunction in congenital anemias such as hemoglobinopathies, particularly with splenic hypofunction or acquired anemias such as megaloblastic anemia. |
| Heinz bodies |
Several grayish, round inclusions visible after supravital staining with methyl crystal violet of the peripheral blood smear, often in the context of bite cells; represent aggregates of denatured hemoglobin. |
Indicative of oxidative injury to the erythrocyte, such as occurs in G6PD deficiency, or less commonly of unstable hemoglobins. |
| Howell-Jolly bodies |
Usually one or at most a few purplish inclusions in the erythrocyte visible on the routine peripheral blood smear; represent residual fragments of nuclei containing chromatin. |
Associated with states of splenic hypofunction or after splenectomy. |
| Schistocytes |
Red blood cells that are fragmented into a variety of shapes and sizes, including helmet-shaped cells; indicative of shearing of the erythrocyte within the circulation. |
Associated with microangiopathic hemolytic anemias, including DIC, TTP/HUS, as well as other mechanical causes of hemolysis, such as prosthetic valves. |
| Spherocytes |
Red blood cells that have lost their central pallor and appear spherical; indicative of loss of cytoskeletal integrity due to internal or external causes. |
Associated with hereditary spherocytosis, autoimmune hemolytic anemia; may also be observed in addition to schistocytes in the presence of microangiopathic hemolytic anemia. |
| Teardrop cells |
Pear-shaped erythrocytes visible on peripheral blood smear; indicative of mechanical stress on the red blood cell during release from the bone marrow or passage through the spleen. |
Seen in a variety of conditions, including congenital anemias such as thalassemia and acquired disorders such as megaloblastic anemia; may also suggest a more ominous process such as myelophthisis (marrow replacement). |
| Burr cells (echinocytes) |
Red blood cells that have smooth undulations present on the surface circumferentially; pathogenesis unknown. |
Indicative of uremia when present on a properly made peripheral blood smear. |
| Spur cells (acanthocytes) |
Red blood cells that have spiny points present on the surface circumferentially; reflective of abnormal lipid composition of red blood cell membrane. |
Most commonly indicative of liver disease when present in significant numbers; also seen in abetalipoproteinemia and in red blood cells lacking the Kell blood group antigen. |
| DIC, disseminated intravascular coagulation; G6PD, glucose-6-phosphate dehydrogenase; HUS, hemolytic-uremic syndrome; TTP, thrombotic thrombocytopenic purpura |
associated with lymphoproliferative and rheumatologic disorders, so these potential underlying conditions should routinely be considered when it is found. Although the cause of a microangiopathic hemolytic anemia is often clear from the clinical setting, it sometimes is a clue to the diagnosis of an occult malignancy, such as metastatic prostate cancer.
CONCLUSION
Anemia may represent a primary hematologic disorder or may represent the manifestation of a systemic process. In the pediatric population, the former tends to be somewhat more common than the latter, and in adults the converse is true. However, in both children and adults, a systematic approach to the evaluation of anemia that includes careful review of historic features, the complete blood cell count, and peripheral smear facilitates an efficient diagnosis and minimizes unnecessary testing.
|


